Has there ever been a worse public policy disaster than the response of almost all governments to COVID-19?
It is now clear that COVID-19, while very infectious, is no more lethal that various influenza epidemics, with an Infection Fatality Rate (IFR) of around 0.1 per cent. As I pointed out in Quadrant in March (“Logic, the First Casualty”), Stanford Professor of Epidemiology John Ioannidis correctly estimated the IFR for Covid as 0.125 per cent, this being the rate at which those infected perish. He also estimated the Case Fatality Rate (CFR) as about 1 per cent, this being the fatality rate of the cases being treated.
These estimates have proven to be accurate and comparable to those for seasonal influenza—and less than the 2 to 3 per cent CFR of the H1N1 influenza virus that caused the 2009 swine flu pandemic and less than the rate initially estimated by the WHO, which has not accepted the lower figure. While swine flu was not as infectious as Covid, governments did not close down societies and economies and trample civil liberties in response to the swine flu pandemic. Why have they with Covid?
This essay appears in November’s Quadrant.
Click here to subscribe
There are several factors that help explain why. Media, both social and conventional, have inflated the perception of risk. Bad news sells, and the madness of crowds can now spread electronically.
The fact that the grim statistics, reported in a daily ritual, have been unreliable has not helped, with increased numbers often simply reflecting increased testing. The reporting of deaths has bordered on negligent, with those positive for Covid being registered as dying from Covid, despite (as the US Centers for Disease Control recently reported) 94 per cent of those dying with Covid having significant comorbidities (an average of 2.6 comorbidities each). Greater funding was made available for a Covid death, so there was an incentive to so record deaths. George Floyd, who died in police custody in Minneapolis, was positive for Covid and would have otherwise been counted as dying from Covid, had other causes not be assigned.
Like George Floyd’s death, Covid became politicised, President Trump’s opponents keen to hold him at fault for every death and every mistake—despite mortalities being highest in Democrat-controlled states like New York and New Jersey, which both have mortality rates adjusted for population double the highest European jurisdiction (Belgium). Trump came under further fire after Bob Woodward revealed in a new book that Trump talked down the risks posed by Covid early in the year so as not to spread undue alarm.
Alarmist modelling by “experts” did not help, when they predicted deaths in the hundreds of thousands or millions in the case of the US. There were fears that those requiring respirators and intensive-care beds would overwhelm health systems. Nowhere have they come close to doing so, but the alarum led to a suspension of diagnostic treatments and elective surgery and caused many not to attend medical providers. This alone will increase mortality down the track.
But there was a reason why Trump was initially more sanguine about the nature of the pandemic: an editorial published online in the New England Journal of Medicine on February 28 written by Anthony S. Fauci (Director, National Institute of Allergy and Infectious Diseases), H. Clifford Lane (National Institutes of Health, and Fauci’s deputy) and Robert R. Redfield (Director, Centers for Disease Control and Prevention, Atlanta). In this paper, the three leading official public health experts correctly identified the correct IFR of 0.1 per cent, about the same as seasonal flu, or a pandemic influenza (similar to 1957 and 1968), rather than SARS or MERS, which had case fatality rates of 9 to 10 per cent and 36 per cent, respectively.
Unfortunately, as pointed out by Dr Ronald Brown in a paper in the Cambridge journal Disaster Medicine and Public Health Preparedness, on March 11, 2020, the US Congress House Oversight and Reform Committee received information from Dr Fauci’s National Institute of Allergy and Infectious Diseases (NIAID) concerning severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and coronavirus disease 2019 (COVID-19) which it causes. Congress was informed that the estimated mortality rate for the coronavirus was ten times that for seasonal influenza, with the reason apparently being (according to Brown) that they confused the CFR with the IFR, an order of magnitude different in both instances. The comparison turned out to be between an adjusted coronavirus CFR of 1 per cent and an influenza IFR of 0.1 per cent. This amplified the sense of panic and “helped launch a campaign of social distancing, organizational and business lockdowns, and shelter-in-place orders”.
In early May, a New York State survey of 1,269 Covid patients recently admitted to 113 hospitals found that most of the patients had been following shelter-in-place orders for six weeks, which raised state officials’ suspicions about social mitigation measures for reducing Covid deaths. Still, polls showed the public believed social distancing helped prevent the spread of the coronavirus. The consequences of this error still persist in policy decisions, and the costs have been stupendous.
A group of 200 economists supported the draconian public health measures, arguing that expense should not discourage the quest to save lives. This, of course, was nonsense, because governments always have to place a value on human life and if they do not do so explicitly, they do so at the margins of the decisions they make. A graduate student of mine in Queensland once told me that government used a figure of $1 million in exercising judgment as to where the limited resources for road improvement should be spent. The accuracy of the figure was of no great consequence, but it allowed competing projects to be ranked, and limited pork barrelling in government electorates. As Nikita Khrushchev once observed, politicians are the same the world over: they promise a bridge even when there is no river.
Adam Creighton, Economics Editor of the Australian, has been playing almost a lone hand in drawing attention to the costs of responses, including opportunity costs, and recently cited an analysis by Martin Lally which put the cost of each year of life saved by the New Zealand lockdown at NZ$8.5 million. This figure was 190 times greater than NZ$45,000 value public health experts had estimated for a year of life before the pandemic.
This kind if cost-effectiveness study is by no means unusual. The gold standard research in this area is probably Tammy O. Tengs et al’s “Five‐Hundred Life‐Saving Interventions and Their Cost‐Effectiveness” in Risk Analysis in 1995. There is also a voluminous literature on the valuation of life by scholars like Kip Viscusi, inferring self-valuations by examining the size of the risk premium people will accept for engaging in occupations which carry a greater probability of death (underground versus surface mining, for example).
The emotional popular response to such thinking is that it is cruel to place a value on human life, but, as Covid has shown, it is more cruel not to. Given that the mean age of Covid deaths in most countries is over eighty (close to the life expectancy in most), a few bonus years comes at the cost not just of younger generations who will eventually have to repay the deficits run up by governments, but also the cost of health care and education forgone in the lockdowns. This does not include, of course, the immeasurable cost of the loss of our civil liberties. Ignoring these costs is heartless, especially when the pandemic presents no greater risk to societies than did the H1N1.
There are some promising portents that these realities are coming to be appreciated. Melbourne University modellers have now warned Victorian Premier Daniel Andrews that their models alone cannot drive policy because they fail to take account of social and economic factors. Andrews’s draconian response to the second wave in Victoria reflects the policy failures in that state, but the wave began to decline before the measures could have taken effect, since the peak was passed before the fourteen-day incubation period that is assumed to be the norm.
Yet Andrews and other governments that committed to such draconian measures (not recommended, it should be noted, by the October 2019 advice of the WHO on how to manage pandemics) have steadfastly ignored contrary evidence that their measures are misplaced and come at enormous cost—one reason why the WHO recommended against them. Andrews now argues, “We must stay the course.”
Why is this so?
The answer lies in the refusal of governments to admit the error of their ways and to steadfastly hold to the course they have set, because to change would be to admit error. It can be summed up with the expression “path dependency”, but it is not just the structural constraints imposed by past decisions that restricts change (and learning), but the attitudes of those governing.
A clear illustration of this can be seen with the different treatment by health officials of two contending drug treatments for Covid.
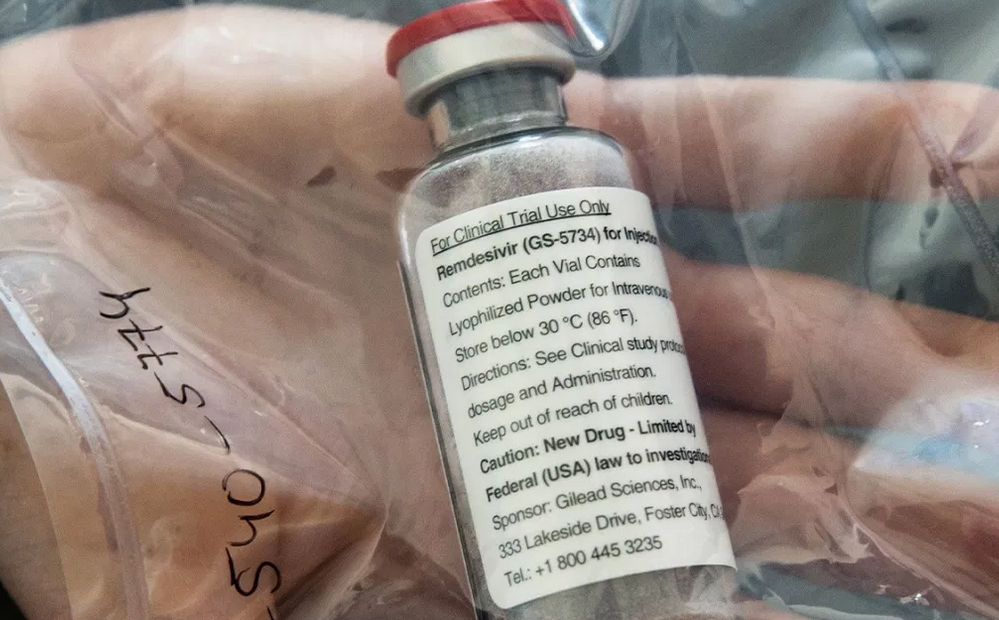
Remdesivir
On April 30 the Financial Review reported that Dr Fauci had announced that the preliminary results from a US government trial (in fact, conducted by National Institute of Allergy and Infectious Disease, the agency he headed) had shown that patients given remdesivir recovered 31 per cent faster than those given a placebo (in eleven days rather than fifteen days). Fauci hailed the results as “highly significant”. To reporters at the White House Dr Fauci likened it to a moment in 1986 “when we were struggling for drugs for HIV and we had nothing”. “It is a very important proof of concept, because what it has proved is that a drug can block this virus,” Dr Fauci said. Fauci did enter a note of caution: the results of the study had not been peer-reviewed, but he expressed optimism that remdesivir would become “the standard of care” for patients with COVID-19.
His optimism was not shared universally. On April 29, the Lancet had published a paper, that was peer-reviewed: “Remdesivir in Adults with Severe Covid-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial”. This Chinese study, the only large, rigorous study for which full data had been made public, reached a rather more pessimistic conclusion than Dr Fauci had over the efficacy of remdesivir:
In this study of adult patients admitted to hospital for severe COVID-19, remdesivir was not associated with statistically significant clinical benefits. However, the numerical reduction in time to clinical improvement in those treated earlier requires confirmation in larger studies.
Moving quickly after Dr Fauci’s endorsement (and ignoring the Chinese study) the NIH’s COVID-19 Treatment Guidelines Panel members decided on May 1 to allow for the emergency use of remdesivir and the Food and Drug Administration granted an Emergency Use Authorisation (EUA). The FDA’s chief scientist, Denise Hinton, stated that the approval was based on “topline data” of the remdesivir study, which meant that the FDA had not had time to fully analyse the results as it would for a conventional approval. She also stated in her letter announcing the EUA that no printed matter related to the drug “may represent or suggest that such products are safe or effective”.
The manufacturer of remdesivir, Gilead Science, moved quickly to donate enough doses for 200,000 patients, and the US government bought another 500,000 – 90 per cent of Gilead’s production of remdesivir for the next three months. Gilead announced it would charge US$2340 for every course of treatment sold to the government, charging private health insurers US$3120. The value of the US contract alone was US$395 million, with more profits to come later though subsequent sales, helped by establishing remdesivir as the “standard of care”. (Gilead has refused to waive its patents.)
This was a good result for Gilead, which had originally developed the drug to treat hepatitis C. It was then tested against Ebola virus disease and Marburg virus disease, but was ineffective for all of these. Before the EUA for Covid it had no commercial application. It had been developed with the assistance of US government finding, estimated by Public Citizen at $70.5 million for overall remdesivir research and development. Remdesivir was essentially a solution looking for a problem.
A study in the New England Journal of Medicine on April 11, sponsored by Gilead, had earlier reported favourable results, and prepared the way for study by Fauci’s institute, but by mid-May, NEJM posted several responses that indicated significant flaws in the calculations of the purported benefit of the drug, the mortality observed in the study and questionable statistical methodology. The authors acknowledged their errors and provided a revised, somewhat lower figure, but NEJM did not retract the paper, as Retractionwatch thought it should have, and simply labelled the new text a “response”.
In Australia, News GP, the newsletter of the Royal Australian College of General Practitioners, reported on June 8 that the National Covid-19 Clinical Evidence Taskforce made a conditional recommendation for doctors treating coronavirus patients to consider using remdesivir to treat adults hospitalised with moderate, severe or critical COVID-19.
Taskforce Chair, Associate Professor Julian Elliott, stated that they had recommended the use of remdesivir based on preliminary data from two randomised controlled trials that had been published on the drug—a “preliminary report” from the trial by Fauci’s institute which had by then been published in NEJM on May 22, and a clinical trial in China published in the Lancet on April 29. This was the same trial that the Financial Review coverage of Fauci’s statement referred to, stating that it had “showed the opposite” conclusion to the US study, yet Elliott was citing it as supporting the use of remdesivir.
This recommendation allowed Gilead to ease remdesivir onto the Australian market, using its “loss leader” strategy, with SBS reporting on June 2 that a spokesperson for Health Minister Greg Hunt had confirmed that it had donated a large number of doses to Australia. (The Task Force recommendation apparently predated this donation, but the original statement is no longer on the Task Force website.) At this stage, the drug was not even approved by the Therapeutic Goods Administration (TGA). Perhaps unsurprisingly, that approval came on July 11.
So remdesivir, a drug with mild known side-effects including possible liver inflammation, nausea and sweating, found an easy passage through regulatory approval.
Hydroxychloroquine
The path followed for remdesivir is in marked contrast to that followed for hydroxychloroquine (HCQ), a generic drug (out of patent) developed originally as a treatment for malaria, but which showed early in vitro promise and was regarded as demonstrating considerable potential. Perhaps unfortunately, it was given endorsement by President Trump in an off-the-cuff remark and became highly politicised. Negative assessment was helped by the publication in the Lancet of a paper suggesting that it had serious side-effects, including heart arrhythmia—a known complication, but one managed in normal use. This paper was soon formally retracted and seems fraudulent, and the side-effects were not an obstacle to its use for many years when prescribed for lupus and rheumatoid arthritis, in addition to malaria.
A study at the Henry Ford Health System based in Detroit considered 2541 mostly older, African-American patients, who were treated early in hospital with HCQ (paired in some cases with azithromycin). This study found that HCQ provided a 66 per cent hazard ratio reduction, and HCQ plus azithromycin 71 per cent compared to neither treatment.
The biggest obstacle HQC faced, however, was probably that it was out of patent and therefore cheap, and it posed a serious competitive threat to remdesivir. In fact, the evidence suggests that HCQ is most effective early in an infection and remdesivir later, so they are probably not competitive, but complementary. What is significant is that once the US NIH’s COVID-19 Treatment Guidelines panel endorsed remdesivir, it turned away from HCQ, as did many researchers.
Three trials were halted: the “Solidarity Trial” (WHO, cancelled on July 4); the ORCHID Study (NIH, discontinued on June 20); and the “Recovery Trial” (Oxford, discontinued on June 5). A “White Paper” published by the Economic Standard in September 2020 drew attention to the shortcomings of several of these studies, such as failing to administer HCQ at an early stage and often together with zinc, which other studies had reported to be effective. The Recovery Trial even administered doses in excess of the recommended 2800mg over six days.
While official science closed ranks against HCQ, with Fauci (for example) stating there was no evidence of its efficacy, studies continued to be undertaken and HCQ continued to be used in many countries. A group of medical researchers established a register of HCQ studies (https://c19study.com) which provides the reference and summaries for seventy-three positive evaluations and twenty-four negative (in addition to meta-analyses, in vitro studies and so on). Of those positive evaluations, fifty-eight have come since Fauci et al closed the official door on HCQ on May 1.
The question arises as to why there has been a closing of the official mind and a later refusal to open it again. One answer might lie in the membership of the NIH COVID-19 Treatment Guidelines panel.
Investigative reporter Sharyl Attkisson reported in a story on the US program Full Measure on May 18 that eleven members of the NIH’s COVID-19 Treatment Guidelines Panel reported links to a drug company, nine of them declaring relationships to Gilead. Seven more, including two of the committee’s leaders, had ties to Gilead beyond the eleven months they were required to disclose. Two were on Gilead’s advisory board, while others were either paid consultants or had received research support and honoraria. The endorsement of remdesivir and veering away from HCQ was perhaps, therefore, not surprising. (A perhaps even more alarming conflict of interest emerged in the United Kingdom, where it was revealed that the Chief Scientific Officer, Sir Patrick Vallance had £600,000 worth of shares in the vaccine maker contracted by the government to make the UK’s coronavirus vaccine.)
But what of Australia’s resistance to accepting HCQ? Gilead Science funds research fellowships to the tune of $300,000 annually, so doubtless enjoys considerable goodwill in the medical community, but the history of recommendations on HCQ reveals another motivation for rejecting the drug for Covid: the preservation of supplies for existing patients for other maladies.
On March 24 the TGA introduced new restrictions limiting the health practitioners who could initiate therapy with HCQ, and then restricted its use under the Pharmaceutical Benefits Scheme on May 8. Then on May 27 the TGA strongly discouraged the use of HCQ to treat Covid except for patients enrolled in a clinical trial. The fear of exhausting supplies because of the possibility HCQ might be efficacious therefore preceded the recommendation not to use it, indicating a strong predisposition and a reason for heightened scepticism.
One of the continuing criticisms of many of the HCQ trials was that they were not the gold standard of drug research: randomised controlled trial (RCT). While such trials are the paragon, many drugs in common usage were approved without RCTs and after observational trials, including tetanus vaccine, insulin, hydrocortisone, tetracycline, Warfarin, Ritalin, Amitriptyline, IV vancomycin and amoxicillin (Economic Standard, 2020: 12).
The use of HCQ for Covid in various countries has, however, provided a quasi-experiment to assess its efficacy. As of September 8, those countries with widespread early use of HCQ show a 78 per cent reduction in deaths per million compared with those with limited early use (Economic Standard, 2020: 16). The spurious withdrawn paper from the Lancet provided an even more emphatic time-series demonstration of the efficacity of HCQ, because its use was suspended in Switzerland on May 27 as a result of this paper and then reinstated in June. Allowing for a twelve-to-thirteen-day lag in deaths, the rate increased by about 10 per cent and then reduced dramatically after recommencement.
Evidence such as this has not convinced regulators to reconsider HCQ, which has been subjected to a more strict burden of proof than remdesivir, which was approved after a single trial (a “proof of concept” study as Fauci put it) before it had been peer reviewed, with a contrary peer-reviewed finding ignored in the US and even counted as evidence in favour in Australia.
Gilead, the maker of remdesivir, has benefited from these decisions, encouraging them by sponsoring some early research (albeit flawed), benefiting from connections with panel members and using a loss-leader marketing strategy to find a market for an antiviral it had developed, assisted by public funds, for which it had no market. Gilead has an interesting history with such regulatory approvals.
In 1999 an antiviral drug developed by Gilead was manufactured and marketed under licence by Roche: oseltamivir or Tamiflu. The licensing arrangement was highly profitable for Gilead, with royalty payments totalling $2.2 billion by 2014. By 2014 Tamiflu had generated more than $18 billion in sales for Roche, with half generated by sales to governments and companies around the world to create stockpiles in preparation for pandemics. The US bought $1.3 billion worth to create a strategic reserve and the UK £424 million for a stockpile. Most doses have never been used. H1N1 swine flu turned out to be not particularly lethal and the recommendations of the WHO and the CDC to build stockpiles in order to be able to “flatten the curve” (sound familiar?) was a very expensive overreaction.
A further problem was that Gilead and Roche had not disclosed all the data on the testing of the product, and this was done only in 2013 after much pressure. When it was disclosed, the efficacy of Tamiflu was rather underwhelming, and several adverse reactions had also not been disclosed. Tamiflu was approved without the RCT that was demanded for HCQ, with one source stating, “If we had that sort of data we would give it primacy, but we don’t live in that world. We needed to use observational data.” An RCT at that time was also considered to be ethically questionable. There is a case for relaxing demands of an RCT in the Covid crisis, perhaps even more so than with Tamiflu, but HCQ was not granted that concession.
Gilead has been criticised previously for its practices, having introduced a hepatitis C drug, Sovaldi, that was priced at $1000 a pill, or $84,000 a course. Gilead raised the ire of AIDS activists over its pricing practices, and they sued in 2016, claiming that Gilead kept a new drug (TAF) on the shelf for several years while it continued to extract patent-protected profits from marketing tenofovir.
There are some green shoots. Finally, on October 1 the Australian reported that the Walter and Eliza Hall Institute in Melbourne would undertake a trial of the efficacy of HCQ for prophylaxis against Covid.
Paths Followed and Not Followed
The path dependencies exhibited with remdesivir and HCQ are markedly different. Both exhibit different standards with initial reasons for preserving HCQ supplies for other uses affecting what has happened since. Past decisions have locked in subsequent decisions, and there has been a lack of learning.
The same has been the case with non-pharmaceutical interventions adopted on an assumption of Covid lethality that was in error—by an order of magnitude. Economic analysis and good policy analysis implores us to let bygones be bygones. But the politics surrounding policy-making works against this. In the face of likely blame attribution for making mistakes, those who have made these decisions are likely to double down on their errors—with costly consequences. The adversarial nature of our accountability mechanisms tends to reinforce this tendency.
Policy-makers are therefore more likely to double down. Cognitive dissonance will help them ignore contrary evidence and the consequences of their actions, and a generally poor media is unlikely to disabuse them of their confidence that they have not made mistakes. Meantime, we all suffer.
References
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., … & Lopez de Castilla, D. (2020). Remdesivir for the treatment of Covid-19—preliminary report. New England Journal of Medicine.
Brown, R. B. (2020). Public health lessons learned from biases in coronavirus mortality overestimation. Disaster medicine and public health preparedness, 1-24.
Davis, M. R., McCreary, E. K., & Pogue, J. M. (2020). That escalated quickly: remdesivir’s place in therapy for COVID-19. Infectious diseases and therapy, 1-12.
Doidge, Norman (2020) ‘Hydroxychloroquine: A Morality Tale.’ Tablet https://www-tabletmag-com.cdn.ampproject.org/c/s/www.tabletmag.com/amp/sections/science/articles/hydroxychloroquine-morality-tale
Dyer, Owen (2020) ‘Cochrane reviewer sues Roche for claiming Tamiflu could slow flu pandemic BMJ 2020;368:m314 doi: 10.1136/bmj.m314 (Published 27 January 2020).
Financial Review (2020) ‘Remdesivir “can block this virus”: Fauci’ Financial Review 30 April. https://www.afr.com/world/north-america/remdesivir-can-block-this-virus-fauci-20200430-p54ohb
Florko, Nicholas, and Damian Garde (2020) ‘With remdesivir, Gilead finds itself at strategic crossroads, with its reputation (and far more) at stake.’ Statnews. 5 May. https://www.statnews.com/2020/05/05/remdesivir-gilead-strategic-crossroads-reputation-far-more-at-stake/
Full Measure (2020) ‘Hydroxychloroquine’ 18 May 2020 http://fullmeasure.news/news/cover-story/hydroxychloroquine
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., … & Nicastri, E. (2020). Compassionate use of remdesivir for patients with severe Covid-19. New England Journal of Medicine, 382(24), 2327-2336.
Gupta, Y. K., Meenu, M., & Mohan, P. (2015). The Tamiflu fiasco and lessons learnt. Indian journal of pharmacology, 47(1), 11.
Hydroxychloroquine Trial https://hcqtrial.com
Jack, A. (2014). ‘Tamiflu:“a nice little earner”.’ BMJ, 348, g2524.
Retractionwatch (2020) ‘NEJM, Lancet place expressions of concern on controversial studies of drugs for COVID-19.’ https://retractionwatch.com/2020/06/02/nejm-places-expression-of-concern-on-controversial-study-of-drugs-for-covid-19/
The Economic Standard. (2020) ‘Hydroxychloroquine And The Burden of Proof’ 22 September. https://hcqwhitepaper.com
Tsirtsakis, Anastasia (2020) ‘Antiviral becomes first recommended treatment for COVID-19’ News GP 8 June. https://www1.racgp.org.au/newsgp/clinical/antiviral-medication-remdesivir-becomes-first-reco
United States et al ex rel Thomas Jefferson v Roche Holdings et al. Case 14-cv-03665-GLR. US District Court for the District of Maryland. Filed 3 Sep 2019, unsealed Jan 2020
USA. National Institute of Health. (2020) COVID-19 Treatment Guidelines Panel. Financial Disclosure. COVID-19 Treatment Guidelines Panel Financial Disclosure for Companies Related to COVID-19 Treatment or Diagnostics. https://www.covid19treatmentguidelines.nih.gov/panel-financial-disclosure/
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., … & Hu, Y. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 395: 1569–78.
 Sign In
Sign In 0 Items (
0 Items ( Search
Search










This has only ever been about their planned great New Reset and a means to defeat Donald Trump, who was standing in their way. In my opinion!
Sadly it has nothing to do with saving lives!